
Eat Less Live Longer?
Can calorie restriction extend human lifespan?
(c) Ian Williams Goddard
Only one intervention has been proven to extend both the average and maximum lifespan of all animal species tested: reducing the consumption of dietary calories, or calorie restriction (CR). [1-2] While widely recommended, exercise and nutritional supplementation have not been shown to extend maximum lifespan. [3-5] Because CR extends maximum lifespan, scientists believe it actually slows the process of aging. CR is therefore used as a means to study the process of aging. [6,7]
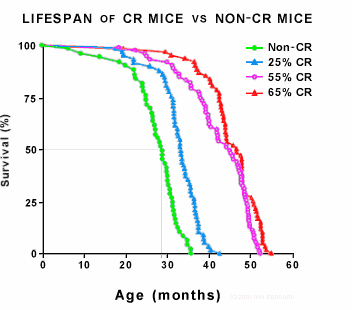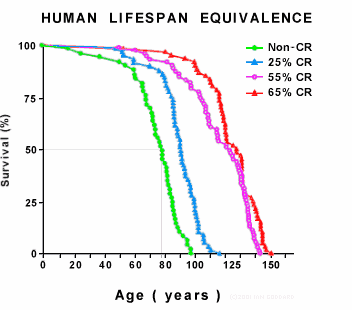
The graph above shows the lifespans of four groups of mice, illustrating the dramatic life extension induced by life-long CR. [8] The first group (green) were controls who ate freely without restriction and define normal lifespan. The other three groups were subjected to different degrees of CR initiated at one month of age, which is equivalent to a 2 year old child. Such early onset CR results in stunted growth and is therefore not acceptable for humans. The results found that more CR resulted in more life extension -- a pattern that holds until CR becomes actual starvation, whereupon it shortens lifespan. [9] The graph is a two-frame animation. The second frame shows equivalent human lifespan.
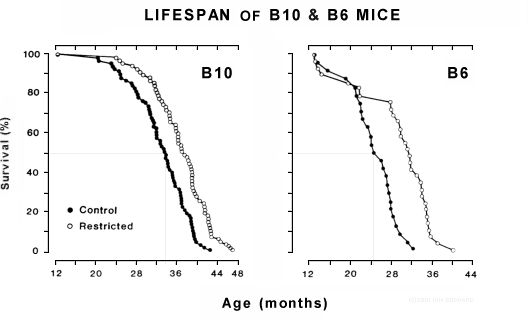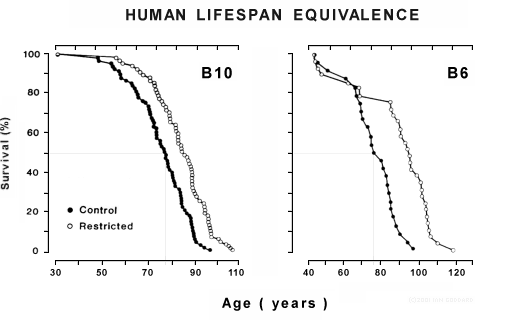
Adult-onset CR: Only adult-onset-CR data are relevant for human consideration, and life extension is less when CR is initiated in midlife, approaching nil when initiated in late life. [10] The next graphs show the lifespans of two long-lived mouse types gradually subjected to 44% (B10) and 27% (B6) CR starting at 12.5 months of age versus controls. B10 mice started CR at a human-age equivalence of 30, while B6 mice started CR at a human-age equivalence of 40. [11] Note: Adult-onset CR extends animal life only when phased in gradually (over a period equivalent to 2.5 years in humans) and when augmented with a nutrient-enriched diet.
CR not only extends the lifespan of laboratory animals but also reduces the incidence of virtually all diseases of aging such as cancer, [12-15] heart disease, [16,17] diabetes, [18-20] osteoporosis, [21,22] auto-immune disorders, [23-25] neurological decline [26-30] and diseases such as Alzheimer's [31] and Parkinson's. [32-34] Those references are linked to abstracts at the National Library of Medicine, please follow them for further details. While CR has failed to extend some cognitive functions in the Fisher-344 rat, [35,36] overall, CR has been shown to dramatically extend both the life and health of all animal species tested to date.
From Mice to Men?
The question that matters is: Will CR do for humans in real life what it does for animals in the lab? Because we humans live so long, no CR lifespan experiments have been conducted on humans. However, if CR can extend human lifespan one would expect to find a correlation between low body weight and longevity, since eating less is associated with lower weight. The fact that such a correlation does exist tends to support the hypothesis that CR will do for humans what it does for other mammals.
While early studies suggested that lower body weight was associated with increased mortality, once researchers accounted for factors such as smoking and illness-induced weight loss, the data showed a correlation between lower weights and increased longevity. [37] Several examples:
* In 1985, the National Institute of Health, Centers for Disease Control, and the Department of Health and Human Services published a "special report" stating: "[S]tudies based on life insurance data, the American Cancer Society Study and other long-term studies, such as the Framingham Heart Study and the Manitoba Study, indicate that the weights associated with the greatest longevity tend to be below the average weights of the population as long as such weights are not associated with concurrent illness or a history of medical impairment." [38]
* In 1993, the Journal of the American Medical Association published a study that concluded: "In these prospective data, body weight and mortality were directly related. After accounting for confounding by cigarette smoking and bias resulting from illness-related weight loss or inappropriate control for the biologic effects of obesity, we found no evidence of excess mortality among lean men. Indeed, lowest mortality was observed among men weighing, on average, 20% below the US average for men of comparable age and height." [39]
* In 1995, a study published in New England Journal of Medicine concluded: "Among women who never smoked, the leanest women ... had the lowest mortality, and even women with average weights had higher mortality. Mortality was lowest among women whose weights were below the range of recommended weights in the current U.S. guidelines. Moreover, a weight gain of 10 kg of more since the age of 18 was associated with increased mortality in middle adulthood. These data indicate that the lowest mortality rate for U.S. middle- aged women is found at body weights at least 15 percent below the U.S. average for women of similar age." [40]
* In 1997, the American Journal of Clinical Nutrition published a study on body weight and mortality stating: "We conclude that when appropriate adjustments are made for effects of smoking and underlying disease, optimal weights [for longevity] are below average in both men and women; this appears to be true throughout the adult life span." [41]
While such studies based on epidemiological data establish correlation, not causation, the weight of these findings among human populations in addition to laboratory proof that CR extends the lifespan of other mammals tends to favor the hypothesis that CR will also extend human lifespan.
Okinawa: Less Calories More Life
The Japanese district of Okinawa has the longest average lifespan in the world [42] and the highest percentage of centenarians -- people living to a 100 or more -- ever documented from reliable records. [43] Consistent with CR-induced life extension, Okinawans also eat up to 40 percent fewer calories than Americans [44] and 17 percent fewer calories than the Japanese average. [45] The caloric intake of Okinawan children is 36 percent below the Japanese recommended intake. [45] And yet, satisfying a necessary ingredient for CR-induced life extension, Okinawans have adequate nutrition. [45]
Not only do Okinawans have reduced mortality, but also consistent with animal CR research, they enjoy reduced morbidity from a range of causes. For example, these findings were presented at the annual meeting of the American Geriatrics Society (2001) [44]:
|
While many factors may contribute to Okinawan lifespan, researchers tend to favor the CR theory as the best explanation. [44] Even without explicit human CR research, available data tends to favor the hypothesis that CR-induced life extension may be a universal effect that applies to all species including humans. Perhaps the next best thing to human research is CR research on primates, which is currently underway.
Primate CR Research
Since 1987, the National Institute on Aging has been conducting a long-term study of CR on rhesus monkeys. In 1999, the NIA researchers stated: "[E]merging data from studies of CR in rhesus monkeys show promise that the model is working in a manner similar to that seen in rodents thereby strengthening the possibility that the well known effects of CR on lifespan, disease, and aging processes may be generalizable to all species." [46]
More recently, I contacted NIA researcher George Roth, who told me: "Morbidity and mortality appear to be lower in CR monkeys." He stated further that this difference from controls is approaching statistical significance. [47] About the NIA study, Modern Maturity states: "The incidence of diabetes ... is greatly reduced in monkeys on a restricted diet. The monkeys also show fewer signs of spinal arthritis, a common condition they share with humans." [48] These monkeys show other signs of reduced aging, such as a prevention of age-associated decline in melatonin levels. [49]
This table shows other bio-markers in the CR monkeys and comparison to findings in CR rodents. [46]
| Findings in NIA Primate CR Study | |
| (-) Body weight | |
| (-) Fat and lean mass | |
| (-) Time to sexual maturation | |
| (-) Time to skeletal maturation | |
| (-) Fasting glucose/insulin | |
| (-) Metabolic rate (short-term) | |
| (*) Metabolic rate (long-term) | |
| (-) Body temperature | |
| (*) or (+) Locomotion | |
| (-) Triglycerides | |
| (+) IGF-1/growth hormone | |
| (-) Il-6 | |
| (*) Wound closure rate | |
| (*) Clonal proliferation | |
| (*) B-gal senescent cells | |
| (-) Lymphocyte number | |
| (*) Lymphocyte calcium response |
While researchers at the Wisconsin Regional Primate Center found different gene-expression changes between CR primates and rodents, [50] the overall body of evidence cited above suggests that CR is doing for primates -- and thus may do for humans -- what it does for all other animal species tested. Considering the long duration of human lifespan, data derived from primate research in addition to human body-weight data and examples such as Okinawa may be as close as we will come to answering the question: Will CR do for humans what it does for all other animals tested?
Discussion
Having reviewed the available data, one might be inclined to consider embarking upon a CR regime. The correlation between below-average body weight and longevity is by itself sufficient to suggest the wisdom of such. But there are several things one must first consider. For example, any CR regime should (a) be implemented gradually over time, (b) include only highly nutritious foods and supplementation to avoid malnutrition, and (c) be supervised from the beginning by a knowledgeable physician.
An article recently published in Scientific American implies that only extreme near-starvation CR will result in appreciable health benefits. [1] However, the data indicate that deriving benefits from CR is a matter of degree, not all-or-nothing. In other words, some CR is likely to result in some health benefits, while progressively more may result in progressively more benefits that fall off only as CR becomes malnutrition, whereupon CR becomes harmful. Merely cutting out junk foods, virtually all of which are high-caloric, by itself could result in moderate CR.
Initiating CR in mice during adulthood extended average lifespan but failed to extend maximum lifespan until researchers implemented adult-onset CR gradually and provided a nutrient enriched diet for the rest of their lives. In the first study to shown that -- illustrated in the second graphs above -- CR was initiated at an incremental level for one month, followed thereafter by a higher level of CR. [11] That one month phase-in equals approximately 2.5 human years. In a more recent study, calorie intake in mice was reduced by 16% for two weeks, followed by 45% CR thereafter. [51] Those two weeks equal around 1.3 human years. A gradual phase-in also makes CR easier, allowing appetite to adjust. In my own experience, CR has increased my enjoyment of food.
Another key to CR is optimal nutrition. Many third-world countries have lower calorie intake and yet do not live longer due in large to inadequate nutrition. Okinawa on the other hand is an example of low calorie intake with adequate nutrition, which researchers believe may be why Okinawans live so long. [44] However, while CR prolongs cognitive functions into old age in animals, researchers at the USDA found evidence of cognitive impairment during CR in obese women [52] probably associated with reduced levels of iron despite the fact that the women were still consuming twice the recommended daily allowance of iron. [53] The same research found significantly improved word recall. [52] But before taking iron supplements consider that excess iron may promote diseases such as cancer [54,55], Alzheimer's [56], and Parkinson's. [57]
While CR appears to be beneficial for many neurodegenerative disorders, immerging data suggest that it may be contraindicated for those with amyotrophic lateral sclerosis (ALS). [58-62] The Calorie Restriction Society lists a number of risks assocaited with CR. The fact that the women doing CR had reduced iron levels despite consuming twice the recommended amount of iron highlights the wisdom of consulting a physician before embarking upon a long-term CR program in order to establish baseline blood measures of as wide a range of nutrients and other health bio-markers as possible. This way the effects of CR on your health can be monitored to detect and correct any deficiencies that might result. Despite the extensive medical literature on CR-induced life extension, some physicians may not be aware of it, especially of its exploratory application in humans. It might therefore be wise to seek out a physician knowledgeable in preventative and anti-aging medicine. [63] It would also be wise to consult resources on CR, such as the website of one of the leading CR experts, Dr Roy Walford. [64]
References
[1] Taubes, G. (2000). The Famine of Youth. Scientific American, June.
[2] Study that discovered calorie restriction extends animal lifespan: McCay CM, et al. (1935). The effect of retarded growth upon the length of life span and upon the ultimate body size. Journal of Nutrition, 10(1), pages 63-79.
Cancer
Heart Disease
Diabetes
Osteoperosis
Auto-immune disorders
Neurological decline
Alzheimer's
Parkinson's
Other topics
[38] NIHNC, CDC, & DHHS. (1985). Body weight, health and longevity: conclusions and recommendations of the workshop. Nutrition Reviews, February, 43(2), pages 61-3.
[42] Investigating the world's longest-live people. Okinawa Centenarian Study.
[44] Okinawa Centenarian Study data presented at the American Geriatrics Society annual meeting, 2001; cited by McCord H, & McVeigh G, (2002). NutritionNews: "Magic" Appetite Shutoff from the Orient. Prevention, January, pages 52-3.
[45] Kagawa Y. (1978). Impact of Westernization on the nutrition of Japanese: changes in physique, cancer, longevity and centenarians. Preventive Medicine, June, 7(2), pages 205-17.
[47] Email response from NIA researcher George Roth (geor@vax.grc.nia.nih.gov), January 1, 2002.
[48] Warshofsky F. (1999). The Methuselah Factor. Modern Maturity, November-December.
[63] Search for a Physician or Practitioner, American Academy of Anti-Aging Medicine.
my journal
my home page